
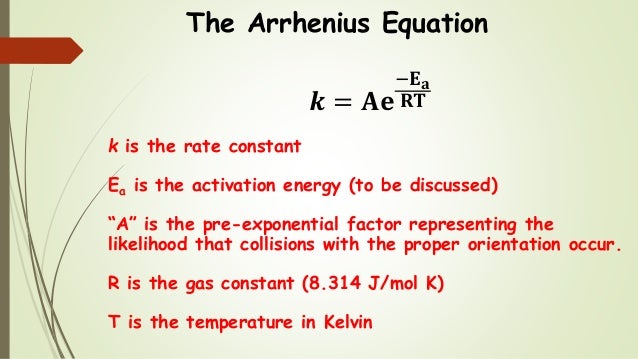
Based on transition state theory, the Eyring equation can also be used to analyze kinetic data: The Arrhenius calculation assumes that the pre-exponential factor, A, is constant, whereas it is actually slightly dependent upon temperature. From the reaction rate constant, the concentration may be calculated assuming a first-order decrease with time. Where k B is the Boltzmann constant (1.38 x 10 -23 J/K), h is Planck's constant (6.626 x 10 -34 J s) and T is the average temperature of the data.Īn equation is provided that allows the reaction rate constant to be calculated at a specified temperature. The pre-exponential factor, A, is used to calculate the activation entropy, ΔS*, from the following equation: Higher values of the activation energy also indicate a greater temperature dependence of the reaction rate.

Low values are often observed for oxidation reactions, while higher values are seen for hydrolyses. Typical values for the activation energy run from 20 to 150 kJ/mol. The slope is multiplied by the gas constant ( R = 8.314 J/K.mol) to give the negative of the activation energy, ΔE*. A is the intercept or pre-exponential factor, and ΔE*/R is the slope. The correlation coefficient is calculated providing a measure of how linear the relationship is the closer the correlation coefficient is to 1.0, the more linear the relationship. The routine fits the data to the Arrhenius equation as a straight line with y values of ln k and x values of 1/T: Entering the time units is only important if you are interested in the activation entropy it has no bearing on the activation energy. On separate lines, enter the temperature (in Celsius) and the associated rate constant, separated by commas, spaces or tabs. The first line of input should be the name of the time units (e.g, hours), followed by a comma and the number of seconds per time unit (e.g., 3600 for hours). In the rate constant and the number of seconds per time unit.įor example, if your rate constant was in hours, then the first
The first line should be the name of the time units used Page performs Arrhenius and Eyring calculations using temperaturesĪnd rate constants as input, and producing activation energy andĮnter the temperatures (in Celsius) and rate constants one per

The calculator converts both temperatures to Kelvin so they cancel out properly.Arrhenius & Eyring Calculations Arrhenius & Eyring Calculations

The Arrhenius Activation Energy for Two Temperature calculator uses the Arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants.


 0 kommentar(er)
0 kommentar(er)
